The Basophil Activation Test (BAT) has gained increasing importance in the field of allergy diagnostics in recent years. This is driven by its higher accuracy and clinical relevance compared to other allergy tests and supported by growing scientific evidence. Beside this, the BAT moved from in house testing protocols to standardized kits, ensuring certified robustness, accuracy, and reproducibility over laboratories and over time.
The implementation of the In Vitro Diagnostic Medical Devices Regulation (IVDR) 2017/746 from May 2022 has been and will continue to be a great challenge for the In Vitro Diagnostic Devices (IVDs) industry, from manufacturers to the laboratories. For BÜHLMANN this has meant: A complete revalidation of the new generation Flow CAST® kit, that has improved quality standards and the demonstration of the clinical performance of the device. We are excited to share some of the results of this validation with you.
This higher blood and cell stability strongly facilitates time management and practicability of BAT
BAT is a functional assay relying on live basophils and detected by the low- to mid throughput flow cytometry technology. Therefore, the appropriate conditions for the specimen storage and the handling of processed samples that BÜHLMANN offers, are crucial for a much wider use of the test in routine applications.
As summarized in the poster presented at the EAACI 2022 congress in Prague, we confirmed previous data that unprocessed EDTA whole blood samples are stable for 48 hours stored at 2-8°C and for 24 hours stored at 28°C until performing the BAT. This allows for facilitated blood sample logistics, to ship blood specimens to laboratories without rigid time constraints. This is a key topic for the BAT to be accepted in the routine test environment.
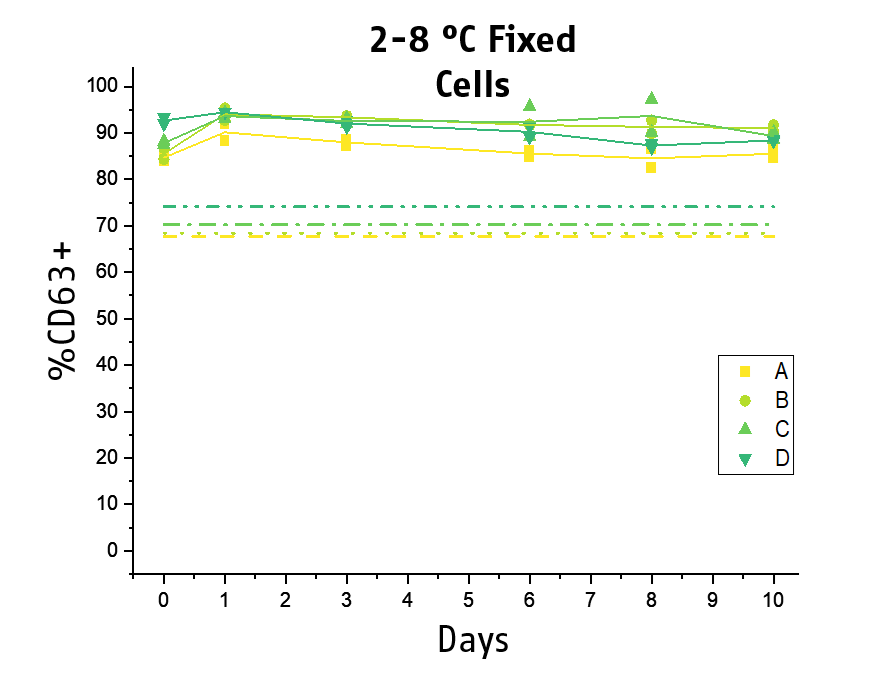
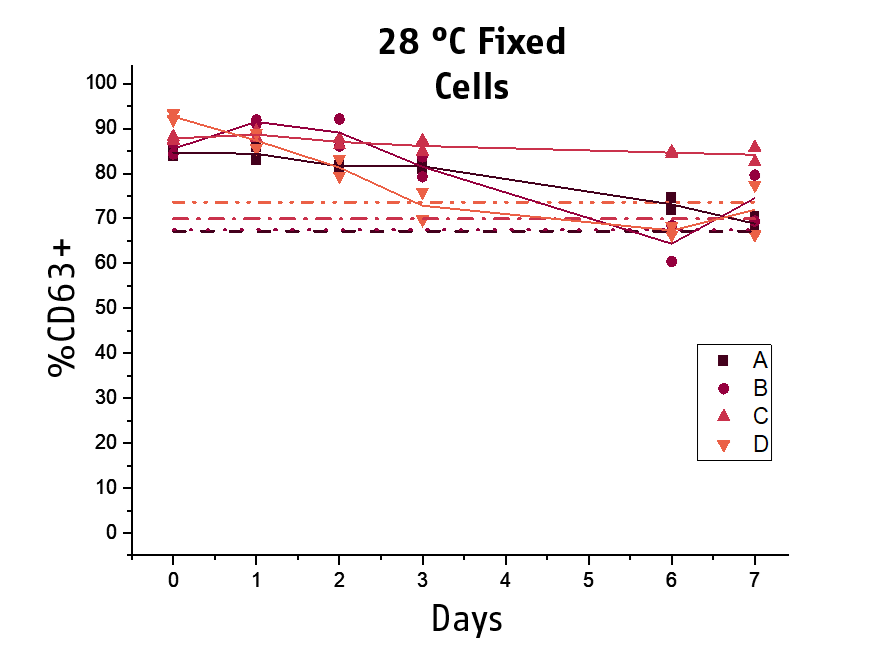
Another key topic is the stability of the stimulated blood. The acquisition of the samples at the flow cytometer can become a workflow bottleneck if laboratories do not have new generation high trough put instruments. Thus, to improve the practicability and time management of BAT testing, we included a stabilizing agent for the processed basophils to our next generation Flow CAST® kit. We could demonstrate that processed and fixed basophils remained stable for the flow cytometry acquisition for at least 10 days at 2-8°C (min. 80% recovery), while for 28°C, 48 hours of stability could be shown.
This allows for subsequent flow cytometry acquisition, facilitating time management and hence practicability of BAT testing for laboratories.
Influence of potentially interfering substances and abnormal blood conditions on the BAT
Another important point that we addressed in the revalidation of the Flow CAST® is the potential of pharmaceuticals or abnormal blood conditions to interfere with the test results. As shown in the poster presented at the EMBRN 2022 meeting in Utrecht, a total of 13 substances were investigated, including eight pharmaceuticals (antiallergic drugs, mast cell stabilizers, leukotriene antagonists, cortisone medication), four abnormal blood conditions (triglyceride, bilirubin, and haemolysis) and one sample additive (K-EDTA). No interferences were detected at the tested concentrations on the stimulation controls anti-FcεRI mAb. This confirms that BAT can be safely performed in individuals who are taking anti-allergic treatments.
The IVDR certification of the next generation Flow CAST® kit is expected to come in spring 2023 while the CAST® Allergens are already IVDR cleared.
Do you want more informations about the next generation Flow CAST® kits? Please contact us.


Social Links