- info@buhlmannlabs.ch
- +41 61 487 12 12
- Home
- Products & Solutions
- Gastroenterology
- Infliximab

Quantum Blue® Infliximab
The biologic drug Infliximab is a therapeutic monoclonal antibody. It acts as an antagonist to TNF alpha, thus effectively blocks the inflammatory process in numerous chronic inflammatory diseases like Crohn’s disease, ulcerative colitis and inflammatory arthritis. The BÜHLMANN Quantum Blue® Infliximab is the first rapid test to measure infliximab trough levels in patient’s serum to allow immediate decision making for potential drug dose adjustments.

Quantum Blue® Infliximab
The biologic drug Infliximab is a therapeutic monoclonal antibody. It acts as an antagonist to TNF alpha, thus effectively blocks the inflammatory process in numerous chronic inflammatory diseases like Crohn’s disease, ulcerative colitis and inflammatory arthritis. The BÜHLMANN Quantum Blue® Infliximab is the first rapid test to measure infliximab trough levels in patient’s serum to allow immediate decision making for potential drug dose adjustments.
Infliximab Trough Level
Over the past two decades great improvements in therapy of chronic inflammatory diseases have been made. The rise of TNFα biologics like infliximab has been a great step forward to ameliorating disease course and keep inflammations at remission levels for prolonged periods of time. Patients with suboptimal drug concentrations have worse outcomes than those with adequate drug levels. Therapeutic drug monitoring (TDM) for infliximab has a great potential for the management of anti TNF therapy.
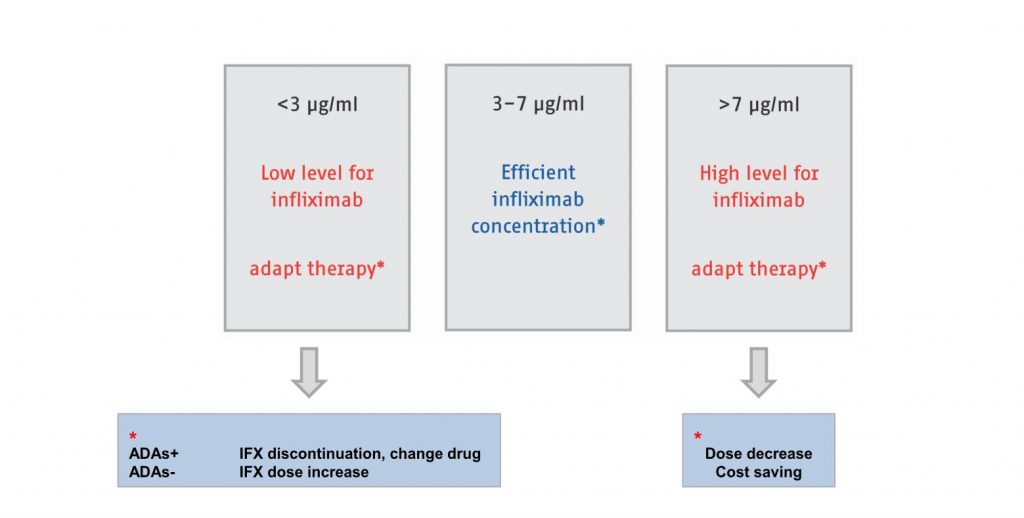
Studies have shown that targeting an adequate infliximab trough level can support guidance of treatment in IBD. An optimal therapeutic window for trough levels seem to be the range from 3 to 7 µg/mL of infliximab in serum.
Adapted from Casteele et al., Gastroenterology, 2015
Quantum Blue® Infliximab
The Quantum Blue® Infliximab assay measures from 0.4 to 20 µg/mL. The target concentrations for trough level interpretation lies well within the linear range of the test. Quantum Blue® Infliximab provides a quantitative result within 15 minutes of incubation time. This offers the possibility to health care providers to act immediately before infusion of the next infliximab dose. Quantum Blue® Infliximab is unique in the diagnostics market, being the only test that can provide fast, reliable TDM for infliximab and it correlates well with the standard routine ELISAs for infliximab level determinations.
Quantum Blue® Infliximab Product Information
| Test | Quantum Blue® Infliximab |
| Order Code | LF-TLIF25/LF-TLIF10 |
| Time to Result | 15 min (approx.) |
| Sample Type | Serum |
| Standard Range | 0.4-20 µg/mL |
| Limit of Detection (LoD) | 0.15 µg/mL |
| Reader Compatibility | Quantum Blue® Reader 3rd generation / Quantum Blue® Reader 2nd generation |

Social Links